Automation solutions that meet the highest demands of the pharmaceutical industry
Tailor-made systems with full documentation, hygiene classification and Ex adaptation
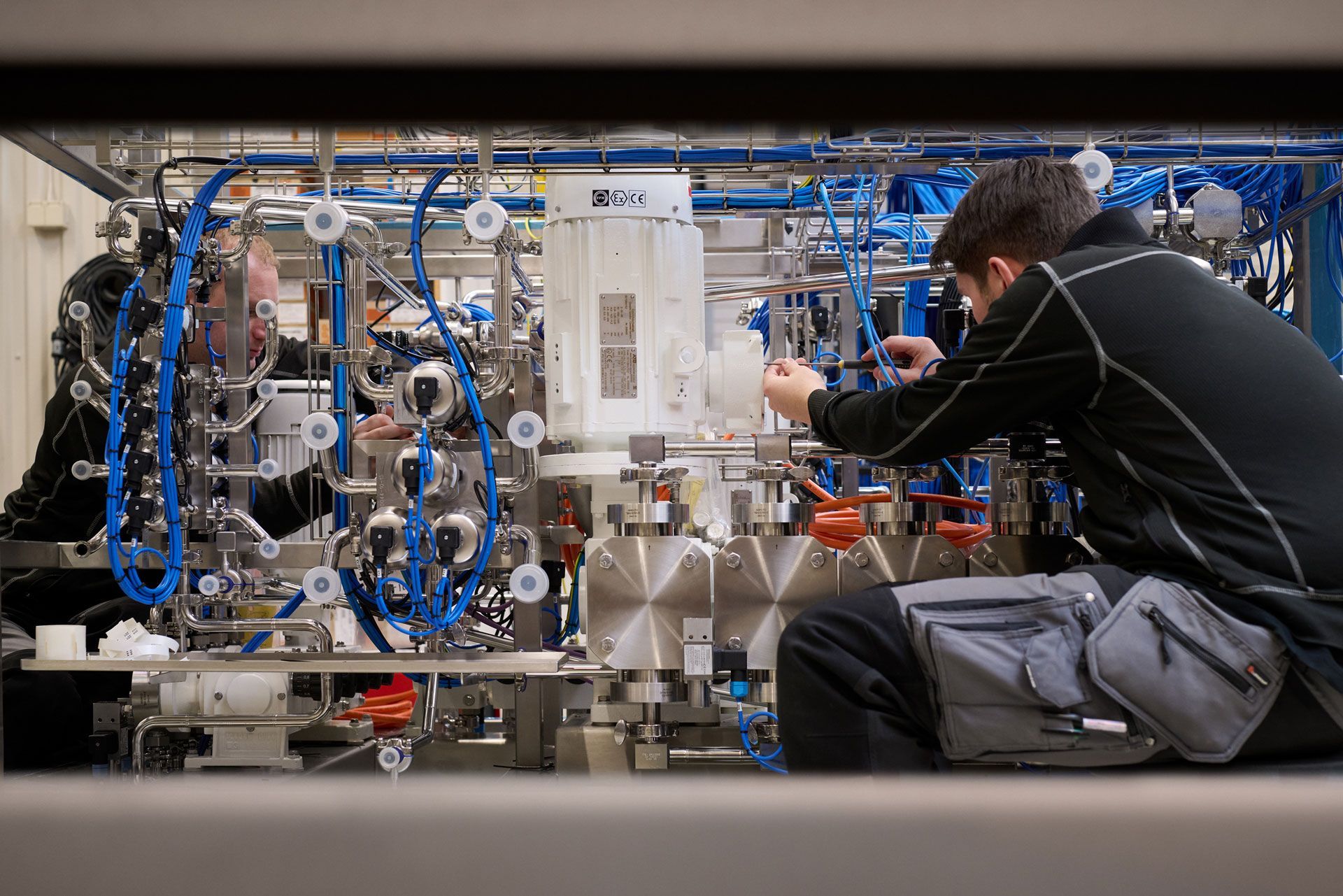
In the pharmaceutical industry, there is no room for compromise. Processes must be repeatable, safe and traceable – every time. JOKRAB has extensive experience in delivering automation solutions that meet the highest demands for hygiene, documentation and reliability. Whether it is dosing, packaging or handling sensitive substances, we build solutions that work in practice and are approved upon review.
Advantages:
- Full hygiene and GMP compliance
- Ex-rating
- No standard solution – everything is customized
- Complete documentation
Example:
Dosing stations, mixing tanks, enclosure systems, bioprocess solutions, filtration systems
About documentation
All our solutions come with comprehensive documentation, including electrical drawings, component lists, risk assessments and – ready for regulatory inspections and internal quality reviews – we ensure you have access to the right information, when you need it.
Do you want to automate a GMP process or modernize an existing cleanroom system?
Get in touch and we'll tell you how we can help you from drawing to validated system.




